Biomechanics is the application of mechanical principles including the bioengineering concepts and the research and analysis of the mechanics to living organisms. In the biomechanical research, by using the engineering methods, the motion pattern of living organisms, how this motion is controlled and to investigate the influence of the interacting structure under the force system which are sourced from different parts of the organism during the mechanical movement activity is tried to be understood. It is also tried to be analyzed the stress-strain components of mechanical loading measured through living or non-living tissue where the treatments are also tested and improved.
Especially for the last 20 years, the biomechanics field has achieved advanced improvements both in academical works and industrial applications. Whether by the view of instrumentation or operation techniques, the new advancements in the dense headings such as, engineering, nanotechnology, computer science, robotics and advanced material science has started to be applied in medical science, eventually the overall work progress in the biomechanics field has accelerated.
As being established in 1999, in the Institute of Informatics the Computational Cardiovascular Biomechanics research group has been working actively under the Computational Science and Engineering Graduade programme with a strong support of computer infrastructure. The group heavily concentrate on the computational fluid dynamics, vessel structure and in vitro tissue modelling.
Research Topics
- Growth and Remodeling of Soft Tissues
- Modelling Human Arterial Network
- Vessel Segmentation and Surface Reconstruction Techniques for Medical Images
- Simulation of Clot Cascading Process in Blood Flow
- Modeling of Deep Vein Thrombosis (DVT)
- Multi-Physics Modeling of Blood Rheology
- Multi-Phase Modeling of Blood Rheology
Research Projects
- Hemodynamic Simulation of Supra Left Ventricular Heart Blood Flow Coupled with Coronary Arteries, Funded by TUBITAK Project Type 1001 and Under the Project Number 124M416, February 2025 - February 2028.
- Computational Hemodynamic Modelling of Prosthesis Heart and Venous, Funded by TUBITAK Project Type 1001 and Under the Project Number 120M671, February 2021 - February 2024.
- Computational Modelling of Deep Vein Thrombosis, Funded by TUBITAK Project Type 1001 and Under the Project Number 117M430, March 2018 - August 2021.
- A Numerical Approach for Growing and Remodelling of Soft Tissues, Funded by ITU BAP, Sept. 2015 – June 2017.
- Blood Flow Simulation in Human Arterial Tree, Funded by ITU BAP, 2014.
I. Fluid-Structure Interaction (FSI) Simulations of Aortic Valve with Immersed Boundary
 Figure: Effect of aortic valve calcification severity on aortic valve dynamics and flow under uniform and helical inflow conditions.
Figure: Effect of aortic valve calcification severity on aortic valve dynamics and flow under uniform and helical inflow conditions.
In this study, we investigated how different levels of localized calcification affect the function of the aortic valve under realistic blood flow conditions. Using advanced fluid–structure interaction (FSI) simulations with the immersed boundary finite element (IBFE) method, we modeled the aortic valve leaflets across multiple calcification grades. These simulations were run under both uniform and physiologically realistic helical inflow patterns coming from the left ventricular outflow tract (LVOT).
Our research specifically focuses on the progression of calcific aortic valve disease (CAVD) and its impact on valve mechanics and flow dynamics. Key findings include:
- Restricted leaflet motion and reduced orifice area as calcification severity increases.
- Elevated wall shear stress (WSS) and altered flow patterns, particularly near leaflet tips and the aortic wall.
- The influence of asymmetric calcifications on redirecting flow and generating complex vortex structures.
- Disturbances in natural helical flow, potentially impacting energy efficiency and cardiovascular health.
- Detailed quantification of transvalvular indices, vortex dynamics, and WSS-based metrics to assess valve performance and disease risk.
To simulate realistic conditions, we implemented 3D geometries based on anatomical references and classified calcium deposits into Grades 3–6, reflecting increasing disease severity. A three-element Windkessel model was also employed to capture dynamic aortic pressure at the outlet.
This work contributes to a deeper understanding of how localized and progressive calcifications alter aortic valve dynamics and provides valuable insights that can support early diagnostics, treatment planning, and surgical interventions for valve-related diseases.
II. Multi-Phase Flow Modeling of Venous Valve with FSI
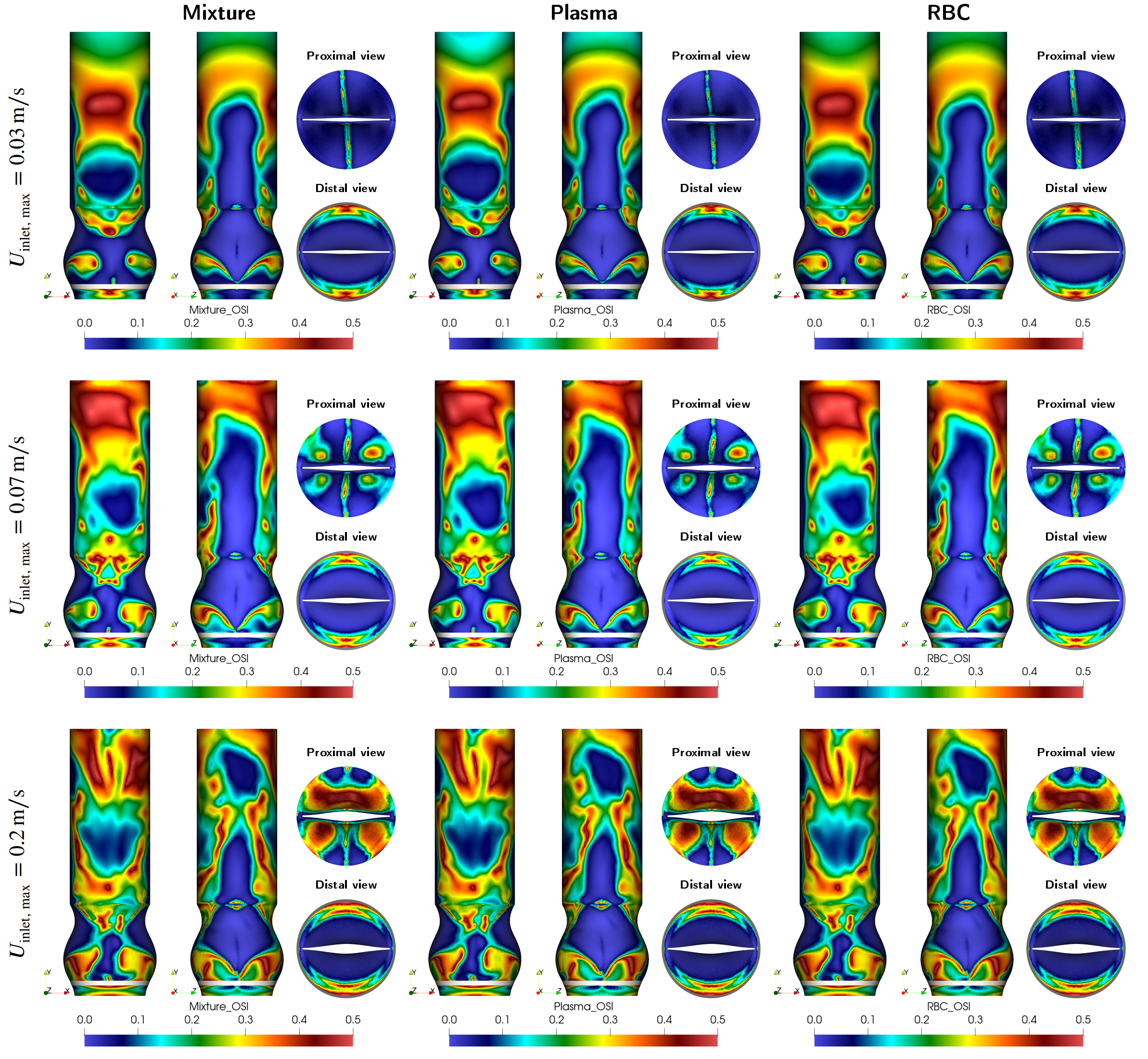 Figure: Variation of the oscillatory shear index for different blood components in deep vein compartment under various physical activities.
Figure: Variation of the oscillatory shear index for different blood components in deep vein compartment under various physical activities.
Venous valves play a vital role in maintaining one-way blood flow in deep veins, particularly in the lower extremities. Dysfunction in these valves can lead to serious conditions like deep vein thrombosis (DVT). Traditional blood flow models often treat blood as a single-phase fluid, neglecting its complex composition. However, blood is inherently multi-phase, consisting primarily of plasma and red blood cells (RBCs), each influencing the flow differently.
In our ongoing research, we implement multi-phase fluid–structure interaction (FSI) modeling to explore how these distinct blood components interact with the biomechanics of venous valves. Our simulations consider realistic physiological flow conditions using advanced numerical techniques, including the Arbitrary Lagrangian-Eulerian (ALE) method. This approach enables the coupling of flexible valve leaflet dynamics with two-phase blood flow to capture more accurate hemodynamic environments than single-phase models can offer.
The use of multi-phase modeling is particularly important for identifying regions susceptible to stasis, oscillatory shear, and other flow disturbances that may contribute to thrombus formation. By incorporating separate behaviors of plasma and RBCs, our work seeks to better reflect the true physiological behavior of venous blood flow, offering a more reliable foundation for future diagnostic and therapeutic developments in vascular medicine.
III. In Silico Hemodynamic Investigations of Coronary Artery
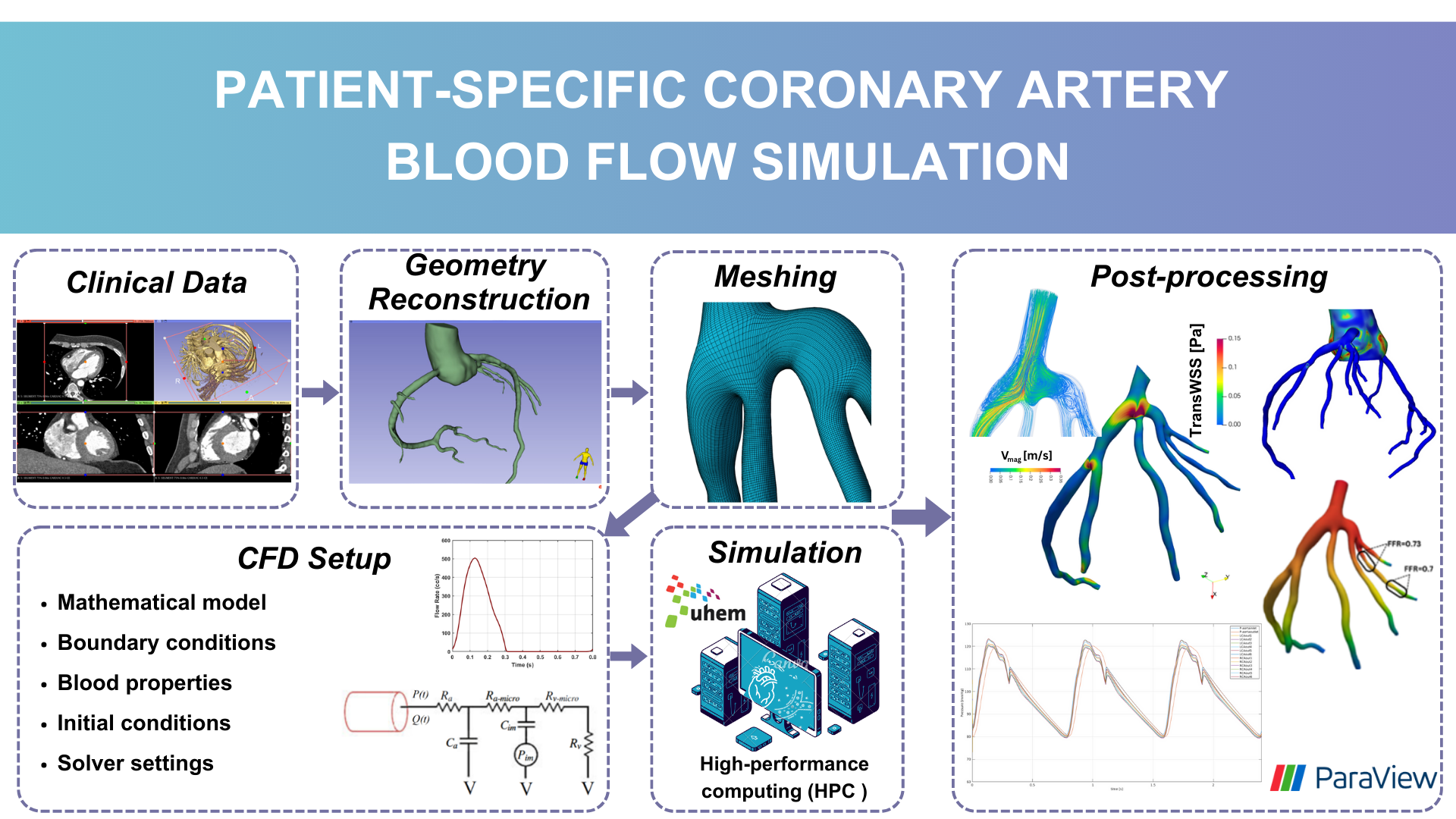 Figure: Workflow of patient-specific hemodynamic simulations.
Figure: Workflow of patient-specific hemodynamic simulations.
The coronary arteries surround the heart, providing blood to the heart muscles, which are essential for delivering oxygen and nutrients. Coronary artery disease (CAD) is a major global health issue, often resulting from plaque formation due to cholesterol accumulation, blood clots, and congenital anatomical abnormalities that can restrict blood flow in the coronary arteries. Hemodynamic analysis is essential for identifying and understanding the problems caused by these disruptions in coronary artery blood flow.
Patient-specific coronary artery 3D geometries have been obtained using non-invasive imaging techniques such as computed tomography coronary angiography (CTCA), followed by segmentation methods to create accurate models of the coronary arteries. Computational fluid dynamics (CFD) or fluid-structure interaction (FSI) simulations are then employed to analyze the hemodynamic behavior of blood flow within these models. Various hemodynamic indexes such as wall shear stress (WSS), time-averaged WSS (TAWSS), transWSS, and fractional flow reserve (FFR) are utilized to investigate the effects of altered flow patterns and identify regions at risk of disease progression.
Figure illustrates the stages involved in Hacer Duzman’s master thesis study, which includes obtaining clinical image data, converting the model from two-dimensional (2D) images to a three-dimensional (3D) geometry, meshing, applying mathematical models and boundary conditions for CFD simulations, determining blood properties, configuring solver settings, and running simulations on high-performance workstations. The final stage encompasses post-processing, visualizing results, and conducting detailed analyses based on these outcomes.
IV. Mechanical Modeling of Venous and Heart Valves
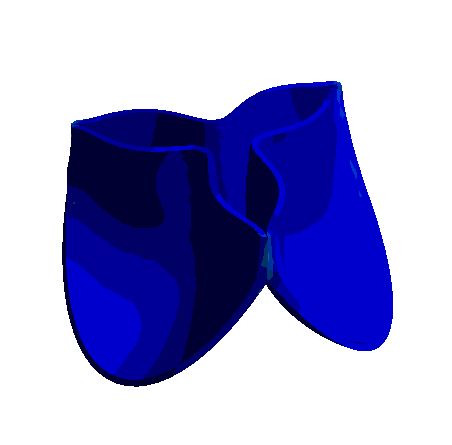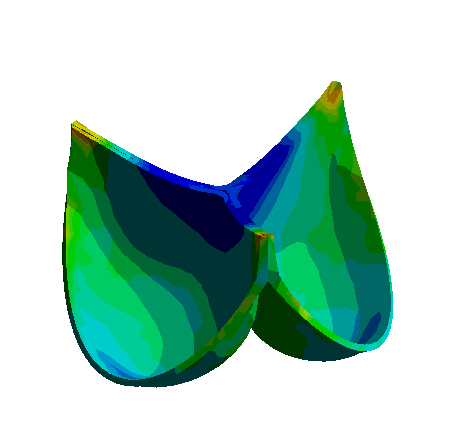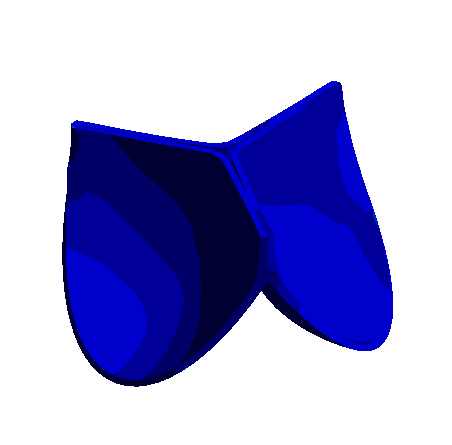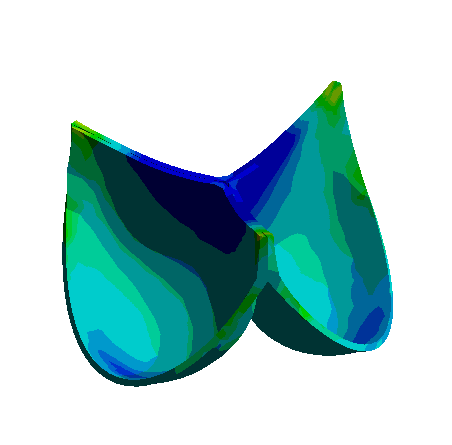 Figure: 3D Representation of Aortic Valve Opening and Closing Dynamics Over a Cardiac Cycle Obtained with Nonlinear Finite Element Analysis.
Figure: 3D Representation of Aortic Valve Opening and Closing Dynamics Over a Cardiac Cycle Obtained with Nonlinear Finite Element Analysis.
The development of bioprosthetic valve designs, particularly for aortic and venous valves, is essential in addressing severe cardiovascular conditions. These valves operate under different physiological environments: aortic valves undergo rapid and large deformations within milliseconds during each heartbeat, whereas venous valves function under lower pressure yet encounter significant biomechanical challenges.
To better understand the mechanical behavior of these valves, finite element analysis (FEA) and fluid-structure interaction (FSI) simulations are employed. These computational methods enable detailed investigation of dynamic valve motion and identification of critical regions, such as zones of stress concentration that could compromise valve durability. Due to the complex geometries, nonlinear material properties, and the transient nature of valve operation, accurate simulation requires fine spatial and temporal discretization.
The research focuses on developing a high-fidelity computational framework that enhances the standard FEA approach by incorporating advanced modeling techniques. This integrated approach aims to improve the predictive capability of simulations, supporting the iterative design and optimization process for creating more durable and reliable bioprosthetic valves.
V. Aortic Valve Design
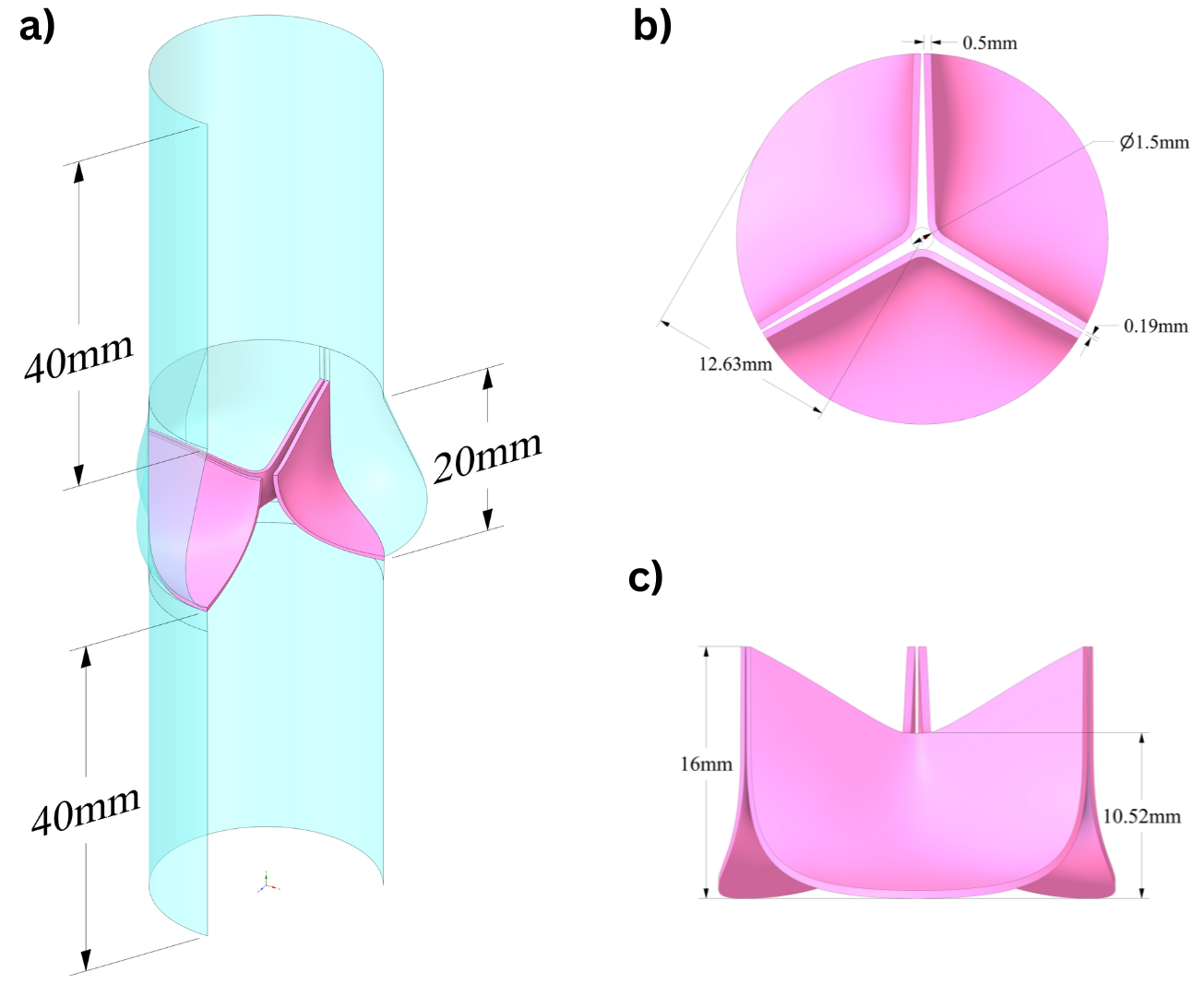 Figure: New aortic valve leaflet and vessel geometry design, a) isometric, b) top, and c) front views.
Figure: New aortic valve leaflet and vessel geometry design, a) isometric, b) top, and c) front views.
Designing functional and durable aortic valves is a key challenge in cardiovascular engineering. Novel valve geometries are often required to improve performance, durability, and patient-specific compatibility. To explore and evaluate such designs, a parametrized modeling approach has bee started to utilized allowing systematic variation of geometric features and rapid testing of design alternatives.
Valve geometries are typically created using advanced CAD tools and Non-Uniform Rational B-Splines (NURBS), which offer flexibility and precision in modeling complex, smooth surfaces. These geometries serve as the foundation for simulation-driven analysis.
Through an iterative design process, each variation is assessed using computational methods, primarily finite element simulations, to evaluate mechanical performance under physiological loading. Feedback from simulations guides further modifications, enabling gradual convergence toward optimal designs.
Additionally, Image-based references from clinical data will be integrated into the design pipeline to ensure anatomical relevance and realism in geometric features in our further studies.